Exploring Experimental Hematology: December 2018 (Volume 68)
Exploring Experimental Hematology: RUNX1 and the Endothelial Origin of Blood
In this issue of Simply Blood, Teresa Bowman is exploring Experimental Hematology by highlighting and deconstructing one of her favorite manuscripts from the ISEH society journal: "RUNX1 and the Endothelial Origin of Blood" by Gao et al.
The functionality of hematopoietic stem cells (HSCs) to replenish and maintain the entire hematopoietic system is the basis for bone marrow transplantation, one of the most well established stem cell-based therapies. Transplantation is curative for many hematologic disorders, but immune-matched donor cells remain limited for many patients. Understanding how HSCs form endogenously during development should provide key insights into expanding donor HSCs from existing sources or generating HSCs from pluripotent stem cells. HSCs first arise during embryonic development through an endothelial-to-hematopoietic transition. Extensive work over the past decade has revealed that not all endothelial cells possess the capacity to form blood cells, and that not all hemogenic endothelial cells can make long-term HSCs. The challenge now is to figure out why.
What to expect from this paper:
In the perspective, “RUNX1 and the endothelial origin of blood,” Speck and colleagues describe how the study of viruses and leukemia led to the discovery of a master regulator of the embryonic formation of hematopoietic stem cells. The work gives a nice historical perspective on RUNX1, starting from the initial work identifying RUNX1 as a transcriptional factor, the identification of its early expression in HSC-forming vasculature, and its function as an initiator of hemogenic endothelial specification. The second half of the paper describes emerging efforts to identify other transcriptional regulators that dictate the differences in hemogenic endothelial cells from distinct sources.
My reason for reading the paper:
My group studies the genetics of HSC formation and regeneration. Nancy Speck is a leader in the field of developmental hematopoiesis awarded the prestigious Donald Metcalf Award from ISEH in 2018. She has been at the forefront of RUNX1 research for most of her scientific career. Her new collaboration with Kai Tan, a noted computational biologist, is addressing a critical question regarding HSC formation:
What drives the heterogeneity of hemogenic endothelium?
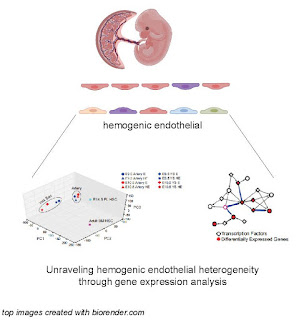
In this perspective, Speck, Tan and colleagues nicely outline the problem and present new gene expression profiling data and transcription factor network analysis striving to uncover why some hemogenic endothelial cells make restricted progenitors and others make pre-HSCs. I enjoyed how this piece put the progress of the field into a historical context and also projected the direction it is moving.
Reasons you should read this paper:
This article is a must read for both novice and experts in the field of hematopoiesis.
1. If you are new to the field, this perspective provides a clear framework of the important questions regarding how HSCs and other progenitors form during development and why the heterogeneity of hemogenic endothelial formation is a critical question in regenerative biology.
2. If you are an expert in the field, this perspective adds new insight into the transcriptional regulation of hemogenic endothelium from two distinct sources, the yolk sac and the arteries. The results presented describe how the source of hemogenic endothelial cells is a main driver of transcriptional differences. Moreover, they provide all their gene expression comparisons in Supplementary Table E1 so you can see the expression pattern of your favorite gene or pathway in yolk sac or arterial hemogenic endothelial cells.
Teresa V. Bowman, PhD
Publications Committee Chair
Assistant Professor, Department of Developmental & Molecular Biology
Assistant Professor, Department of Medicine (Oncology)
Albert Einstein College of Medicine
Bronx, NY
What to expect from this paper:
In the perspective, “RUNX1 and the endothelial origin of blood,” Speck and colleagues describe how the study of viruses and leukemia led to the discovery of a master regulator of the embryonic formation of hematopoietic stem cells. The work gives a nice historical perspective on RUNX1, starting from the initial work identifying RUNX1 as a transcriptional factor, the identification of its early expression in HSC-forming vasculature, and its function as an initiator of hemogenic endothelial specification. The second half of the paper describes emerging efforts to identify other transcriptional regulators that dictate the differences in hemogenic endothelial cells from distinct sources.
My reason for reading the paper:
My group studies the genetics of HSC formation and regeneration. Nancy Speck is a leader in the field of developmental hematopoiesis awarded the prestigious Donald Metcalf Award from ISEH in 2018. She has been at the forefront of RUNX1 research for most of her scientific career. Her new collaboration with Kai Tan, a noted computational biologist, is addressing a critical question regarding HSC formation:
What drives the heterogeneity of hemogenic endothelium?
In this perspective, Speck, Tan and colleagues nicely outline the problem and present new gene expression profiling data and transcription factor network analysis striving to uncover why some hemogenic endothelial cells make restricted progenitors and others make pre-HSCs. I enjoyed how this piece put the progress of the field into a historical context and also projected the direction it is moving.
Reasons you should read this paper:
This article is a must read for both novice and experts in the field of hematopoiesis.
1. If you are new to the field, this perspective provides a clear framework of the important questions regarding how HSCs and other progenitors form during development and why the heterogeneity of hemogenic endothelial formation is a critical question in regenerative biology.
2. If you are an expert in the field, this perspective adds new insight into the transcriptional regulation of hemogenic endothelium from two distinct sources, the yolk sac and the arteries. The results presented describe how the source of hemogenic endothelial cells is a main driver of transcriptional differences. Moreover, they provide all their gene expression comparisons in Supplementary Table E1 so you can see the expression pattern of your favorite gene or pathway in yolk sac or arterial hemogenic endothelial cells.
Teresa V. Bowman, PhD
Publications Committee Chair
Assistant Professor, Department of Developmental & Molecular Biology
Assistant Professor, Department of Medicine (Oncology)
Albert Einstein College of Medicine
Bronx, NY


.png)


Comments
Post a Comment